Zhantu Ye, Zhen Shen, Yilin Zhang, Félix Manuel Rosado-García, Jiawei Ye , Yuefei Ji*, Xin Yu, Mingbao Feng*
Water Research
https://doi.org/10.1016/j.watres.2024.122269
Published: 22 August 2024
Abstract
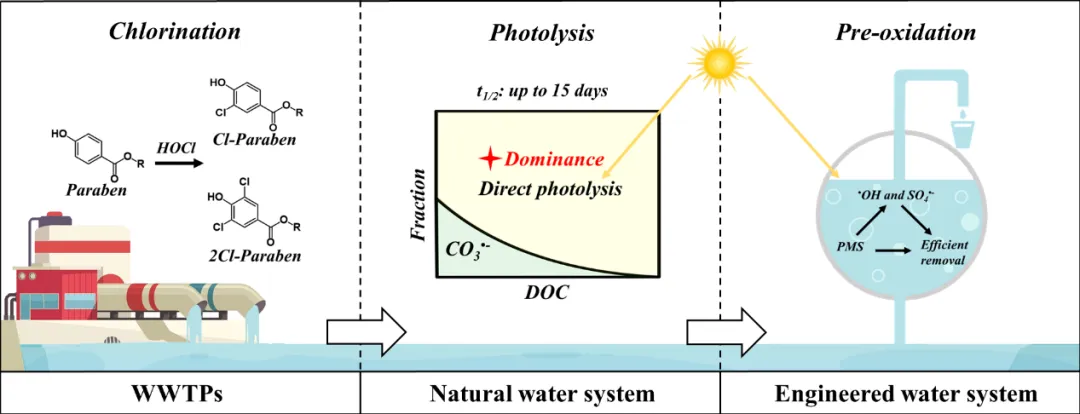
Parabens are classified as emerging contaminants in global waters, and the ubiquitous emergence of their highrisk chlorinated products generated from chlorine-based wastewater disinfection has attracted increasing attention. However, rather limited information is available on their photofate after discharging into surface waters, and their degradation behavior after solar-based engineering water treatment is unclear. Herein, the reactivity of four chlorinated parabens with different photochemically produced reactive intermediates was measured. Quantitative contribution analysis in abating such compounds showed the dominance of direct photolysis in sunlit natural freshwaters. Introducing a technical solar/peroxymonosulfate (PMS) system could greatly improve the removal of chlorinated parabens. The economic analysis suggested that chlorinated parabens exhibited a minimum value of economic input as 93.41-158.04 kWh m-3 order-1 at 0.543-0.950 mM PMS. The high-resolution mass spectrometry analysis of the degradation products suggested that dechlorination, hydroxylation, and ester chain cleavage were the dominant transformation pathways during photolysis and solar/PMS treatment. Furthermore, the in silico prediction indicated severe aquatic toxicity of certain products but enhanced biodegradability. Overall, this investigation filled a knowledge gap on the reactivity of chlorinated parabens with diverse reactive transients and their quantitative contributions to the photolysis and solar/PMS treatment of emerging micropollutants in water.